The hundreds of receptors that give us our sense of smell have been found to have important roles in other parts of the body, and the prospect of targeting them with drugs is growing.
A coloured transmission electron micrograph of an olfactory neuron (orange).Credit: Steve Gschmeissner/SPL
People are estimated to be able to discriminate between anywhere from 10,000 to more than one trillion different smells. In the early 1990s, our understanding of how that can be possible took a big step forward.
By then, it was already known that odours stimulate neurons in the nose by activating a selection of G-protein-coupled receptors (GPCRs) — varieties of which are found in the membranes of most, if not all, mammalian cells. But nobody knew the identity of the olfactory-focused GPCRs — or, crucially, how many different receptors were required to achieve mammals’ immense powers of smell.
In April 1991, Linda Buck and Richard Axel, molecular biologists at Columbia University in New York City, proposed an answer. Buck extracted messenger RNA (mRNA) from the olfactory epithelium in rats and probed it for clues to find which genes were expressed there. She found a huge family of genes that encoded GPCRs1. Buck and Axel hypothesized that the genes represented hundreds of different olfactory receptors.
Subsequent work confirmed that these GPCRs were directly activated by known odours. It is now known that rodents have around 1,000 such genes, and humans have more than 400. Buck and Axel won the 2004 Nobel Prize in Physiology or Medicine for uncovering the molecular foundations of the sense of smell.
But one element of their 1991 study1 has aged poorly. The final figure in the paper claims that the genes encoding the newly identified olfactory receptors are expressed only in the nose. According to Hanns Hatt, an olfactory physiologist at Ruhr University of Bochum in Germany, that is “wrong for sure”.
At the turn of the millennium, Hatt proposed that olfactory receptors were, in fact, expressed in various non-nasal tissues. Many researchers were sceptical, he says, and dismissed the possibility of such receptors being functionally meaningful. Hatt, however, was undeterred. Since 2003, he has published dozens of papers on extra-nasal olfactory receptors.
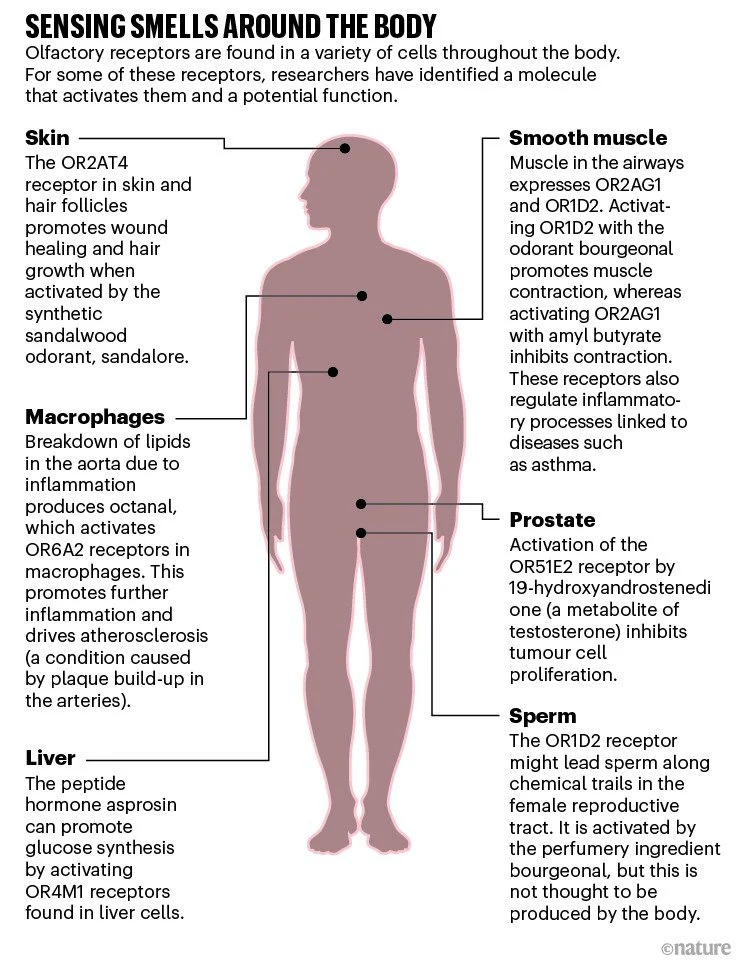
It is now widely accepted that olfactory receptors have important roles beyond the nose. Some contribute to kidney and lung physiology, others to metabolic control, and one might regulate hair growth and wound healing (see ‘Sensing smells around the body’). The receptors are also expressed on some cancerous cells and, in January, one receptor was reported to drive atherosclerosis — a condition caused by plaque build-up inside arteries2. “This is really the very beginning of what I think will be a significant field,” says Klaus Ley, an immunologist at the La Jolla Institute for Immunology in California, who led the atherosclerosis study.
As more researchers begin to investigate extra-nasal olfactory receptors, drug developers are taking note of their therapeutic potential. There are many challenges to overcome — not least the limited pharmacological understanding of these possible drug targets. But some people consider the growing interest in these curiously located receptors to be long overdue. “It’s a huge frontier,” says Stuart Firestein, a physiologist at Columbia University. “I don’t really understand why it’s not been exploited more.”
Seminal work
The possibility that olfactory receptors function beyond the nose was raised remarkably soon after Buck and Axel’s landmark paper. In January 1992, a team led by Marc Parmentier, a molecular biologist at the Université Libre de Bruxelles in Belgium, reported that they had found abundant mRNA for several members of the olfactory receptor family in the testes — sperm cells, specifically — while looking for potential receptors for the hormone thyrotropin3. The researchers speculated that the receptors might help sperm to follow a chemical trail to an egg — and even wondered if blocking the receptors could lead to a new type of contraceptive.
Throughout the 1990s, these data remained a side note; most researchers studying the receptors, Hatt included, focused on understanding their olfactory function. But at the end of the decade, Hatt began to search for human olfactory receptors beyond the nose. “We found them in each human cell type in which we looked,” he says. Soon afterwards, Firestein and his team found mRNA for around 30–110 different olfactory receptors in each of a variety of non-olfactory mouse tissues4. But despite the potential implications of these results, extra-nasal olfactory receptors continued to attract minimal attention. “I was surprised at how little interest there was,” Firestein says.
Hatt felt that for people to take notice, he had to show not only that these receptors were all over the body, but also that they were functional. To do so, his team went back to where it had all started and began to examine an olfactory receptor present in sperm that they could successfully express in a cell line. They screened a mixture of known odour molecules against the receptor, now known as OR1D2, and showed that it was activated by bourgeonal — a chemical with a floral scent reminiscent of lily of the valley5. When bourgeonal bound to the receptor, it produced a surge of calcium inside the sperm cell. And when the group exposed free-swimming sperm to a source of bourgeonal, the cells accumulated around it in a dose-dependent manner.
Their results supported the idea that ova, or some other component of the female reproductive tract, might release chemicals that activate these receptors to help male germ cells to find female ones. That chemical is unlikely to be bourgeonal — mammalian cells are not known to secrete it. Because olfactory receptors can be activated by multiple molecules, there could be a compound produced inside the body that serves this function, but so far a viable endogenous activator has not been identified.
In the years since, Hatt and his group have found olfactory receptors in numerous cancerous cells. For example, in 2009, the researchers showed that a component of essential oils could activate an olfactory receptor known as OR51E2 found on cancerous prostate cells6. Activating the receptor suppressed cell division, suggesting a potential means of keeping such tumours in check. The same might be true of colorectal cancer, whereas in others, such as breast cancer, olfactory receptors are instead being explored as potential biomarkers for malignancy.
Hans Hatt is an olfactory physiologist at Ruhr University of Bochum in Germany.
Hatt’s group also found olfactory receptors in smooth muscle cells in the lungs and in heart cells; activating the receptors affected how the cells contracted. And a receptor activated by a woody-smelling odorant from sandalwood is present in skin cells and hair follicles. In excised samples of human skin, stimulating the receptor promoted wound healing and hair growth7. This work has led to a small trial of a shampoo for people with stress-related hair loss8.
The main constraint on Hatt’s exploration of olfactory receptors around the body has been the fact that many of them are orphan receptors — there are no known molecules that activate them. Because identifying activating chemical messengers is intensive work, without guaranteed success, his laboratory has favoured studying receptors with known activators — leaving hundreds of extra-nasal receptors uncharacterized.
Just by chance
Although Hatt has deliberately pursued olfactory receptors beyond the nose, the field’s expansion has often been more serendipitous, driven by people with no interest in olfaction until they unexpectedly encountered the receptors in various tissues. “We were initially looking at something else entirely,” says Jennifer Pluznick, a kidney researcher at Johns Hopkins University School of Medicine in Baltimore, Maryland, who first encountered olfactory receptors as a postdoc in the mid-2000s.
Pluznick was running a screen of all the genes with altered expression in a model of kidney disease. Surprisingly, some of the starkest changes affected olfactory receptors. “I wasn’t even sure I could trust these data,” she says. “These are supposed to be kidney cells, and I’m getting olfactory receptors coming up.” But encouraged in part by Hatt’s work on sperm cells, and having contacted Firestein for advice, she decided to investigate further. “The more we thought about it, we were like, they are really just G-protein-coupled receptors,” she says. “The name throws people off.” (See ‘Giving receptors a bad name’.)
In 2009, Pluznick published a paper describing six olfactory receptors that are present in the kidney9 — along with the intracellular signalling proteins the receptors operate through. Pluznick then predicted how stimulating GPCRs of this kind would affect specific types of kidney cell and went looking for the function of each receptor.
Four years later, Pluznick and her colleagues showed that one olfactory receptor called Olfr78, found in certain mouse kidney cells, was activated by short-chain fatty acids (SCFAs) such as acetate and propionate10. They then demonstrated that SCFA stimulation mediated the release of the hormone renin, which leads to vasoconstriction.
Jiaojiao Xu works on olfactory receptors in Pluznick’s lab at Johns Hopkins University in Baltimore, Maryland.Credit: Mackenzie Kui
Pluznick also points out that there is a known source of SCFAs in the body: intestinal microorganisms produce the compounds as metabolic by-products, which are then absorbed into the blood11. It is not clear whether plasma SCFA levels are high enough to fully activate these receptors, or why exactly blood pressure would be linked to the gut microbiome, she says. However, it is a plausible model of how renal olfactory receptors might be regulated.
Pluznick now runs a lab dedicated to studying chemosensory receptors in the kidney and cardiovascular system. Among her most recent projects is a study of another SCFA-regulated olfactory receptor that could regulate blood pressure, as well as an orphan olfactory receptor that regulates sugar reabsorption by the kidney12 and has been linked to diabetes in mice.
Search for a source
Atherosclerosis specialist Ley also had an accidental introduction to olfactory receptors. He has been exploring how immune cells, including macrophages, drive inflammation of the aorta for more than 20 years. In 2015, he ran a screen of all the genes expressed by various subpopulations of human macrophages. “We stumbled on these olfactory receptors,” he says. “Of course, we didn’t know anything about them.”
The olfactory biologist he contacted was sceptical, Ley says. But seeing that around 100 of the more than 400 known human olfactory receptors can be expressed by macrophages, Ley began to investigate.
He chose to focus first on the olfactory receptor Olfr2, because it had a known activator: octanal, a compound with a citrus smell. Additionally, there was a known inhibitor: citral, which has lemony scent and is abundant in lemongrass. Ley and his team demonstrated that activating Olfr2 increased levels of intracellular calcium in certain macrophages and caused them to release an inflammatory messenger known to accelerate atherosclerosis2.
To assess whether octanal was the endogenous activator of this process, Ley looked for a plausible source. Food and the microbiome were ruled out, and nobody he asked knew of an enzyme that produces octanal. But, Ley’s team discovered that the breakdown of cellular lipids in an inflamed aorta can produce significant amounts of octanal — creating a positive feedback loop of inflammatory signalling. The team subsequently found that injecting octanal into mice leads to rampant atherosclerosis2. In mice engineered to lack functioning Olfr2, however, octanal did not bring on the disease.
Physiologist Yiguo Wang at Tsinghua University in Beijing had a rather different introduction to olfactory receptors. Instead of finding an olfactory receptor expressed somewhere other than the nose and searching for its function, he began with a function — specifically, glucose synthesis in the liver — and went in search of a receptor.
In a 2019 study, Wang and his team suppressed 728 different GPCRs, one-by-one, in a human liver-derived cell line and tested whether any of them affected glucose synthesis13. Among the receptors that had the biggest effects was the olfactory receptor OR4M1. Decreasing expression of OR4M1 (or the mouse equivalent) inhibited glucose synthesis — suggesting that the receptor normally boosts sugar production when activated.
Knowing that a ligand for the receptor must be present in the serum they used to grow the liver cells, Wang and his team soon found the culprit: asprosin, a peptide hormone that had been discovered in 2016.
This was the first demonstration of an olfactory receptor being activated by a peptide hormone — a genetically encoded signalling molecule that mammals make and release in a controlled manner. A second example of a peptide activating an olfactory receptor was provided earlier this year by a study involving researchers at Shandong University in China14. The discoveries raise questions about the evolution of these receptors — including whether the family of genes that code for olfactory receptors evolved originally to detect external chemical signals or internal ones.
“The more we find these receptors in other tissues,” says Firestein, “the more I’m at least mildly convinced that they might not have started out as olfactory receptors.” Notably, although the gene family is huge in mammals, it is much smaller in other tetrapods, and smaller still in fish, in which it originated. The ancestral receptors might have evolved first in internal tissues, then been co-opted for olfaction when they started to be expressed in sensory structures. From there, Firestein says, “it was quite easy for them to duplicate and expand and mutate and become this very large family of receptors”.
Resolving this biological question will take many years of dedicated evolutionary research. But the peptide discoveries might have more practical implications in the short term for those seeking to develop therapies aimed at olfactory receptors.
Clinical targets
Although numerous researchers now see potential in targeting extra-nasal olfactory receptors with drugs, “these are difficult targets at the moment”, says Antonella Di Pizio, a computational pharmacologist at the Leibniz Institute for Food Systems Biology at the Technical University of Munich in Freising, Germany.
Di Pizio expects olfactory receptors to ultimately be good drug targets — they are GPCRs after all, which are targeted by around 35% of currently approved drugs. However, odorant molecules do not typically have properties that would make them successful drugs. They are generally small, hydrophobic and volatile, and activate olfactory receptors only at fairly high concentrations. “They are very different from drugs that reach our body,” Di Pizio says.
Powerful drugs aimed at olfactory receptors — such as activators or blockers — will need to have good bioavailability across the body, and higher affinity for their receptors than most odorants. The discovery that peptides can bind to olfactory receptors could therefore be significant. These molecules can bind tightly: the affinity of asprosin for Olfr734, for example, is around one-thousand-fold greater than olfactory receptors’ usual affinity for odorants.
As well as improving receptor pharmacology, work must also be done to confirm which receptors are medically viable targets. Some with appealing functions in one tissue might be ruled out by unwanted effects on others. In other cases, the effect of stimulating or blocking a receptor might just be too weak; Wang thinks this probably rules out targeting asprosin receptors to modulate glucose metabolism. Pluznick is hesitant about the commercial applications of her original blood pressure work, too, but she has hopes that emerging targets will have clinical applications.
Ley, however, has already filed a patent for the idea of targeting the human equivalent of Olfr2, known as OR6A2, to treat atherosclerosis, and is seeking commercial partners. He is now investigating further olfactory receptors and their connections to macrophages and inflammation, and has begun work on potential applications in fatty liver disease, the eye disease uveitis and cancer. “You could imagine this to be relevant in all kinds of diseases,” he says.
What was once a biological oddity, overlooked by researchers of all kinds, is now a vibrant field. Hatt, whose lab is winding down, says that he is constantly asked to review papers involving extra-nasal olfactory receptors. “They’re now accepted in the scientific world,” he says. “Many groups are now jumping on the topic. I’m very happy.”
Giving receptors a bad name
The terminology used to describe olfactory receptors found in non-nasal tissues might have contributed to the lack of attention they have received.
Since their tentative discovery, olfactory receptors expressed in non-nasal tissues have mostly been referred to as ‘ectopic’ olfactory receptors. But, ectopic — which comes from a Greek word meaning ‘out of place’ — refers to the presence of tissues, cells or proteins found in abnormal, usually pathological, locations. “‘Ectopic’ means the receptors are always referred to like a side effect, something not normal,” says Di Pizio.
Consequently, many researchers in the field want to change the terminology used to describe these receptors. ‘Extra-nasal’ represents one straightforward and descriptive solution, and is what Hatt has chosen to use in his most recent papers. Di Pizio has proposed the term ‘ecnomotopic’, from Greek words meaning ‘out of the usual place’. Pluznick, has used this term in her latest papers, although she concedes it’s a bit of mouthful. In fact, she is considering whether the field should have made a cleaner break from the past.
When she started presenting her work at meetings, Pluznick says that people would frequently approach her afterwards to tell her that they, too, had seen olfactory receptors in their cells of interest, but they had ignored them because it didn’t seem right. Pluznick would tell them that she nearly dismissed them, too. She wonders if a name that did not tie these receptors to their olfactory function might have encouraged more research. “If they were named ‘chemosensory GPCRs’, or something like that,” she says, “perhaps they would be better studied.”
Nature 606, S14-S17 (2022)
doi: https://doi.org/10.1038/d41586-022-01631-0
References
Buck, L. & Axel, R. Cell 65, 175–187 (1991).
Orecchioni, M. et al. Science 375, 214–221 (2022).
Parmentier, M. et al. Nature 355, 453–455 (1992).
Zhang, X. et al. Proc. Natl Acad. Sci. USA 101, 14168–14173 (2004).
Spehr, M. et al. Science 299, 2054–2058 (2003).
Neuhaus, E. M. et al. J. Biol. Chem. 284, 16218–16225 (2009).
Busse, D. et al. J. Invest. Dermatol. 134, 2823–2832 (2014).
Jimenez, F. et al. J. Cosmet. Dermatol. 20, 784–791 (2021).
Pluznick, J. L. et al. Proc. Natl Acad. Sci. USA 106, 2059–2064 (2009).
Pluznick, J. L. et al. Proc. Natl Acad. Sci. USA 110, 4410–4415 (2013).
Pluznick, J. Gut Microbes 5, 202–207 (2014).
Shepard, B. D. et al. Sci. Rep. 6, 35215 (2016).
Li, E. et al. Cell Metab. 30, 319–328 (2019).
Cheng, J. et al. Cell Metab. 34, 240–255 (2022).